States of Matter
Most of us were taught that there are three states of matter: solids, liquids, and gases.

Solids are rigid and have a fixed shape and volume. Liquids have a fixed volume, but are not rigid. Instead, they take the shape of their container. Gases are not rigid, nor do they have a fixed shape or volume. Gases take the shape of their container, and spread out to fill the available volume.
Scientists now recognize a fourth natural state of matter called plasma. Plasma is ionized gas, and while it is not commonly found on Earth, it may be the most common state of matter in the universe. You may have seen the popular video demonstrating how to create plasma using grapes and a microwave.
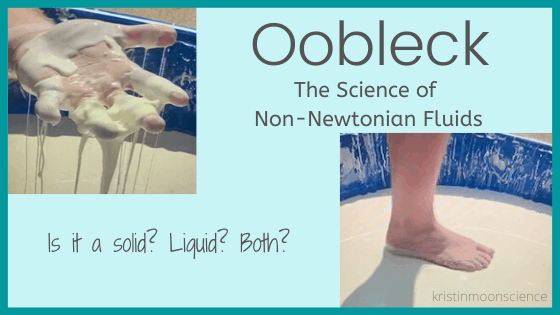
In addition to materials that are easily defined as either solid, liquid, or gas, there are also substances that aren’t so easily categorized. Oobleck is one such substance.
Oobleck, named after the Dr. Seuss book Bartholomew and the Oobleck, simultaneously has properties of both a solid and a liquid. It is a type of substance called a non-Newtonian fluid.
Newtonian and Non-Newtonian Fluids
Newtonian Fluids
Before we can discuss what a non-Newtonian fluid is, it makes sense to understand what a Newtonian fluid is.
Most of us are familiar with the brilliant scientist Isaac Newton. Not only did he invent calculus, he also revolutionized the study of physics with this pioneering work describing the properties of forces. At some time in your life, you’ve likely encountered Newton’s three laws of motion (which I wrote about here).
What is less widely known is that Newton also studied the properties of fluids (liquids). Specifically, he examined the property of liquids known as viscosity. Simply, viscosity describes how easily a liquid flows. A fluid with high viscosity resists flow, while a fluid with low viscosity flows easily.
Consider tap water and honey. If you were to pour each liquid from a container, the tap water would flow quicker and more easily than the honey. We would say that the honey was more viscous than the tap water.
Through his work, Newton concluded that the viscosity of a liquid was dependent on the liquid’s temperature. As the temperature of a liquid increases, its viscosity decreases, and vice versa. Consider honey again. Have you ever tried to pour honey that was cold? Tough, huh? Kind of like that expression, “Slower than molasses in January.” Liquids that display a direct correlation between temperature and viscosity are considered Newtonian fluids.
Non-Newtonian Fluids
Non-Newtonian fluids, like oobleck, don’t follow the same “rules” as Newtonian fluids. Instead of responding to temperature, the viscosity of a non-Newtonian fluid changes with the amount of pressure applied.
Oobleck is a non-Newtonian fluid known as a pressure-thickening fluid (because shear is another word for pressure, these fluids are often called shear-thickening fluids). In the absence of pressure, oobleck looks and feels like a liquid. But when pressure is applied to it, it looks and feels like a solid. If you slowly push your finger into a container of oobleck, your finger will glide in until you touch the bottom. But if you pound on the surface of the oobleck quickly with your fist, the oobleck will resist your fist and it will feel like you’re hitting a soft solid.
How does this happen? Believe it or not, scientists are still trying to figure out what happens to oobleck and other shear-thickening fluids at a molecular level to cause it to behave this way. The simplest, most straightforward explanation I have found comes from the February/March 2017 issue of ChemMatters from the American Chemical Society:
“When a stress is applied slowly to a shear thickening fluid, the polymer chains have time to move out of the way and rearrange themselves, so the viscosity is not affected. But if a quick stress is applied, the polymer chains do not have time to rearrange. Instead, they become entangled, assuming a solid-like consistency, as the viscosity greatly increases. Imagine many cars trying to quickly leave through one exit in a parking lot. If everyone is in a hurry, the cars will become ensnared in a traffic jam. But if the traffic exits slowly, there will be time for each car to leave in an orderly fashion.”
“No Hit Wonder: D30!”. Rohrig, Brian. ChemMatters, February/March 2017.

Oobleck isn’t the only shear-thickening non-Newtonian fluid. In fact, the human body contains such a non-Newtonian fluid. The synovial fluid that coats the knee and elbow joints is a shear-thickening non-Newtonian fluid. Under normal conditions, synovial fluid has low viscosity which allows for easy movement of the joint. But if you bump your knee or elbow, the pressure causes the fluid to thicken, cushioning and protecting your joints.
Another type of non-Newtonian fluid behaves quite differently. The viscosity of shear-thinning fluids decreases as pressure is applied.

Ketchup is an example of a shear thinning non-Newtonian fluid. I’m sure you’ve experienced trying to pour ketchup from a bottle when it all seems stuck on the bottom. What did you do? More than likely, you used your hand to pound on the bottom of the bottle. By applying pressure to the ketchup through that action, the ketchup becomes more fluid and less viscous and easily pours from the bottle.
Shaving cream is another example of a shear-thinning fluid. When shaving cream comes out of the can, it appears to be a soft solid, But rub it between your fingers and it thins into a liquid. Quicksand is also a shear-thinning non-Newtonian fluid. As pressure is applied to quicksand, it becomes more fluid. So if you ever find yourself stuck in quicksand, avoid thrashing about as that will only cause you to sink faster.
Hands-On Fun with Non-Newtonian Fluids
Oobleck is surprisingly simple to make. All you need is cornstarch, water, and a container. I will warn you that the process can get messy, so I advise making and playing with oobleck outside. When you are done, DO NOT POUR OOBLECK DOWN THE SINK! It could easily form a solid plug in your pipes. Instead, dispose of the oobleck in a container or sealable bag and throw it in the trash.
Oobleck Recipe
In a bowl, add two cups of cornstarch. To the cornstarch, add one cup of water. You can use a spoon to mix, but it may become too hard to stir. I recommend using your hands. Continue mixing until your mixture reaches the consistency of honey. You may find you need to add more cornstarch or water. You can scale this recipe up or down, but try to keep the ratio one part water to two parts cornstarch. You can add food coloring, if desired.
Now what? Just have fun with it!! So many things to explore:
- Grab a handful of it and squeeze it. Open your fist. What happens?
- Quickly smack the oobleck with your hand or with a hammer.
- Put some oobleck in a see-through container with a lid. Shake it or hit it against a wall or hard surface. What happens?
- Try rolling some oobleck into a ball.
Here are some other ideas you can try:
I hope you take the time to explore non-Newtonian fluids by making oobleck. People of all ages are amazed by its properties!
Related Posts:
A Crack in the Mystery of ‘Oobleck’—Friction Thickens Fluids
Physicists foil classic oobleck science trick by Science News for Students
If you’re looking for fun, engaging high school science classes with opportunities for hands-on exploration, check out the courses I have to offer here: High School Science Courses Taught by Dr. Kristin Moon Currently I offer high school biology, chemistry, physics, and anatomy and physiology,





great information, pinning for my lad’s use later this year. 🙂
I’m glad you enjoyed it!
This was awesome. Great information. Great explanation and examples.
Glad you enjoyed it!! Now that the weather is warmer, I plan to make an even bigger batch of Oobleck in my kiddie pool!
Fascinating facts, very well explained and great visuals too.
Thanks
I’m glad you liked it! Let me know if there’s something you’d like me to cover in a future post!