In my many years as a science teacher, I’ve heard plenty of people complain about chemistry.
Some even go as far as to say that they hate chemistry. I don’t think they hate chemistry. After all, everything in the world is built on chemistry. In my opinion, people think they hate chemistry because they don’t understand it.
While it is true that understanding chemistry may not come as intuitively as understanding other science topics like biology, it is my belief that everyone is capable of learning chemistry if taught properly.
One reason chemistry may seem hard to understand (or appreciate) is because it deals with atoms and molecules—objects indescribably small and hard to visualize.
Chemistry also comes with its own vocabulary, and many of the terms used in chemistry can be confusing.
I like to think of myself as a science ambassador, and my mission is to help people of all ages understand (and learn to love) science. To that end, I’m answering some common questions I’ve been asked about different science topics.
Today, I will tackle some chemistry terms that often lead to confusion as I explain the differences between atoms, elements, ions, molecules, and compounds.

What is the difference between an atom and an element?
Atoms are the basic building blocks of matter.

Atoms are the building blocks that all matter is made of.
So what is matter?
Matter is anything that has mass and takes up space (has volume).
You are likely most familiar with the 3 states of matter: solids, liquids, and gases, but there are other states of matter as well (including plasma, Bose-Einstein condensates, and non-Newtonian fluids)
Look around you: all that you see (other than energy) is matter. Your chair, your pet, your beverage and its container, and even the air you’re breathing is matter. And atoms are the building blocks of all matter.

You’ve likely seen a diagram of an atom represented as in the picture on the left. This is actually not the most accurate representation of what an atom looks like, but it serves my purpose here. Atoms are made up of smaller particles called protons, neutrons, and electrons.
Protons and neutrons are located in the center–or nucleus–of an atom, while electrons are in constant motion outside of the atom’s nucleus.
An element is a type of atom
An element is a type of atom. To date, we know of 118 different elements, and these are what we see depicted on the modern periodic table. In other words, there are 118 different types of atoms in the universe.

So every block you see on the periodic table represents a different element, and each element is a different type of atom.
Hydrogen is the 1st element on the periodic table. Hydrogen atoms* have 1 proton and 1 electron. Oxygen is the 8th element on the periodic table. Oxygen atoms have 8 protons, 8 neutrons, and 8 electrons.
One fact that I love shocking my chemistry students with: of the 118 elements on the periodic table, only 94 are found in nature. Where did the other 24 elements come from?
They were created in the lab! Isn’t that incredible?
How did they do it? You can learn more here:

What is the difference between an atom and an ion?
This is another great question!
Atoms are made up of 3 subatomic particles: protons, neutrons, and electrons.
Two of the subatomic particles carry an electric charge. Each proton is positively-charged, while each electron is negatively-charged.
Even though atoms are made up of charged particles, an atom as a whole carries no net charge. How is that possible?
In a neutral atom, the number of positively-charged protons and negatively-charged electrons is the same. A lithium atom has 3 protons contributing a charge of +3, but it also has 3 electrons contributing a charge of -3. The positive and negative charges cancel each other out.
So what is an ion? An ion is an atom that has gained a net charge.
Why?
Because it has either gained or lost electrons.
You see, while protons and neutrons are fixed within an atom, electrons are free to move. They can move both within an atom as well as from one atom to another. (The ability of electrons to move from atom to atom is the basis of electricity).
When an atom loses one or more electrons, it loses the negative charges associated with those electrons. Consequently, an atom that has lost electrons will have more positive charges than negative charges, since it will have more positively-charged protons than it has negatively-charged electrons. We see this happen often with metallic elements.
When an atom gains one or more electrons, it gains a net negative charge since it has more negatively-charged electrons than it has positively-charged protons. This happens commonly with atoms of nonmetal elements.

Positively-charged ions are called cations and negatively-charged ions are called anions. A special type of chemical bond called an ionic bond often forms between a cation and an anion. Ionic bonds are formed by the opposite electrical charges of the positively-charged cation and the negatively-charged anion.

Sodium chloride (NaCl), commonly known as table salt, is held together by ionic bonds between sodium (Na) cations and chlorine (Cl) anions.
What is the difference between an atom and a molecule?

Recall atoms are the basic building blocks of matter, and that each element on the periodic table represents a different type of atomic building block.
While it is possible to have single atoms of an element, most of the time, elements combine with other elements to make something new.
When two or more atoms combine, we call that a molecule.
One analogy I use with my chemistry students: an atom is a building block much like the letters of the alphabet are the building blocks of our written language. We can combine atoms to form molecules just like we can combine letters to make words.

Water (H2O) is a molecule made up of 2 hydrogen atoms and 1 oxygen atom held together by chemical bonds.
Hydrogen peroxide(H2O2) is also a molecule made up of hydrogen and oxygen, but it is made up of 2 hydrogen atoms and 2 oxygen atoms.
That seemingly small difference between H2O and H2O2 is what gives the two substances (water and hydrogen peroxide) very different properties.
Each item in the picture below is made up of only 3 building blocks: the elements carbon, hydrogen, and oxygen. But we can all agree that the substances are very different from one another.

This explains how we are able to have the incredible diversity of substances in our universe with only 118 different building blocks.
You can learn more in this video:

What is the difference between a molecule and a compound?
So many people use these terms interchangeably so it can be hard to understand the difference.
A molecule is formed when 2 or more atoms of any element are held together by chemical bonds. A compound is a molecule in which two or more atoms of different elements are held together by chemical bonds.
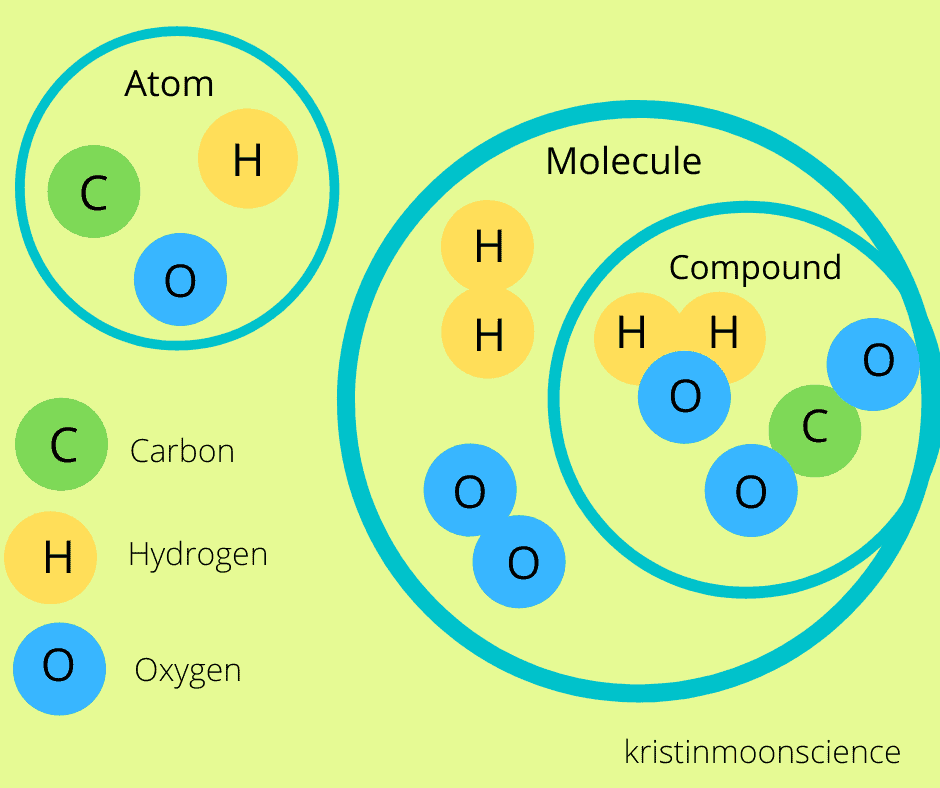
Did you spot the difference?
In the atmosphere, nitrogen is found as a molecule of two nitrogen atoms bound together to form the dinitrogen molecule (N2). N2 is a molecule since it contains 2 atoms held together by a chemical bond, but it isn’t a compound since the two atoms in the molecule are of the same element.
Water (H2O) is both a molecule and a compound, since it has atoms of different elements (hydrogen and oxygen) held together by chemical bonds.
You can review the difference between a compound and an element in this video:

Why are molecules depicted in different ways?
The final topic I’ll address in this post is why molecules and compounds are often depicted in different ways. The reason is because different formats can give us different information about the molecule.

In the diagram above, we see the molecule glucose represented in 3 different ways.
On the left, we see the molecular formula of glucose. From the molecular formula, we can learn what elements combine to make glucose as well as how many atoms of each element are present. From the molecular formula, we see that glucose is made up of 6 carbon (C) atoms, 12 hydrogen (H) atoms, and 6 oxygen (O) atoms.
However the molecular formula tells us nothing about how those elements are joined together. Are the carbon atoms bond to other carbon atoms? What about the hydrogen or oxygen atoms?
To learn how the atoms within an element are bound, we would consult a structural formula of glucose which is shown in the center of the diagram. In a structural formula, we can see not only what types of elements are present and in what number, but we also can see which elements are bound together. We see that the glucose molecule takes on a ring structure made primarily of carbon to carbon bonds. We can also see how each oxygen and hydrogen atom is attached to the molecule.
Finally, we see the glucose molecule depicted as a “ball and stick” model on the far right of the diagram. A ball and stick model gives us information about how the elements and chemical bonds of the molecule are arranged in 3 dimensional space. In high school and college chemistry, students learn that a molecule’s shape (or geometry) directly affects many of its physical properties, such as whether the molecule is polar or nonpolar.
.
I hope this post has helped you understand the difference between the related but distinct terms used in chemistry so that you’re better able to explore this fascinating subject!
If you’d like to learn more about the 118 different elements or explore how the modern periodic table is organized, check out my post: The “Secrets” Revealed in the Periodic Table
While individual atoms and molecules are too small to see at home, with the mole concept, you can measure out grams of water, sugar, and salt and know EXACTLY how many molecules of each substance you have. The mole concept is easily one of my favorites, and you can learn all about why it’s so useful in this post: What is the Chemistry Mole?

If you’re interested in learning more about how electrons move from atom to atom, you can explore static electricity with hands-on experiments while you learn why static cling is so much worse in the cold winter months in this post: Why Static Electricity Seems Worse in Winter
Finally, if you have a student looking for hands-on chemistry labs that are more than simple “kitchen science” but can still be done at home, check out my new Chemistry Lab Video Course. It covers topics included in a first year chemistry course and includes a whole year’s worth of labs. Students can perform the labs themselves, or simply watch the video labs and use my measurements to perform the calculations. Learn more here:
*Many elements exist as different isotopes. Different isotopes of an element have the same number of protons and electrons but differ in the amount of neutrons they possess. Different isotopes of an element behave the same chemically, but have different atomic masses. In this example, I refer to the most abundant isotopes of each element: 1-Hydrogen and 16-Oxygen.
Related Content
Check out my full-year high school science courses: Biology, Chemistry, Physics, and Anatomy and Physiology
pHET: an Excellent Resource for Learning Math and Science
Top Picks for Virtual Labs and Simulations
Chemistry for Your Life Podcast
Chemistry in its Element Podcast







Nice representation and easily understood. Thank you
This was a very helpful resource! Thank you!
Thank you so much!!
Thank you for taking the time to clearly explain these fundamental chemistry concepts. As someone who has struggled to grasp chemistry in the past, I appreciate how you’ve broken down the differences between atoms, elements, ions, molecules and compounds. Your analogies like comparing atoms to letters and molecules to words really help cement the distinctions. I also love that you’ve anticipated common student questions about the mole concept and molecular depictions – your answers help demystify what previously seemed confusing. This is a wonderful chemistry primer that makes these building blocks of matter very accessible. Your passion for imparting scientific knowledge comes through beautifully. I feel much more confident in my basic chemistry comprehension after reading this!